The complete guide to Sinner’s Circle and industrial parts cleaning optimisation

Realizing the benefits of
taking TCO into consideration
for industrial cleaning
Table of Contents
Industrial cleaning and Sinner’s Circle – small changes have a big impact
When it comes to industrial cleaning, many businesses simply focus on getting the job done. The cheapest chemical cleaner, the longest wash cycle, or the highest temperature setting may seem like smart choices in the short term. But in reality, these straightforward decisions often come back as higher costs in other parts of the process: more downtime, shorter bath lifetime, quality issues, and even machine damage.
The truth is simple: small changes in your cleaning setup can have a big impact on both results and costs. At the heart of understanding this lies the Sinner’s Circle, a model that shows how four parameters—chemistry, temperature, time, and mechanics—work together to create an effective cleaning process.
Optimising these factors doesn’t just improve cleanliness; it helps reduce hidden costs and lower your total cost of ownership (TCO). In other words, understanding the science of cleaning is key to achieving better results with fewer resources.
In this guide, we’ll explain what the Sinner’s Circle is, reveal the hidden costs behind “cheap” cleaning, and show you how smarter cleaning strategies lead to more sustainable, cost-efficient production.
Want to know if your cleaning process could be more efficient?
Understanding the Sinner’s Circle in parts cleaning
First introduced in the 1950s by German chemist Dr. Herbert Sinner, the Sinner’s Circle is a model that explains how four parameters determine the outcome of any cleaning process:
-
Chemistry – the cleaning solution used and its concentration
-
Mechanics – the physical action applied, such as spraying, brushing, ultrasound, or agitation
-
Temperature – the heat level applied to support cleaning efficiency
-
Time – the length of the cleaning cycle or exposure
In every parts cleaning process, these four parameters interact to determine whether you achieve consistent, efficient results — or end up with hidden costs and rework.
The principle is simple but powerful: if one parameter is reduced, another must be increased to maintain the same cleaning effect. For example, if you choose to run a wash bath at a lower temperature to save energy, you may need to extend the cleaning time or adjust the chemistry concentration to achieve the same level of cleanliness.
In industrial cleaning, this balance is often overlooked. Many companies default to “more is better” — higher temperatures, longer cycles, stronger detergents — without considering how these adjustments impact cost, sustainability, or equipment. The Sinner’s Circle reminds us that cleaning is not about maximising a single parameter, but about optimising the balance between all four.
When applied to industrial cleaning and degreasing, the Sinner’s Circle provides a framework for improving results, extending bath lifetime, and reducing operational costs. The right balance of industrial cleaning chemicals, and process parameters ensures not only cleaner components, but also a more stable and sustainable production environment.
Even small process adjustments can lead to significante improvements in cost and sustainability
The parameters of parts cleaning
Each cleaning process relies on the four parameters of the Sinner’s Circle — chemistry, temperature, time, and mechanics. Together, they define the quality, efficiency, and cost of your cleaning results. Understanding how each parameter affects the others helps you fine-tune your process for optimal performance and lower total cost of ownership (TCO).
Let’s take a closer look at each one.
1. Chemistry – the heart of cleaning
The chemistry you choose is the foundation of every cleaning process. In industrial parts cleaning, the right cleaning chemistry determines how effectively oils, lubricants, and other contaminants are dissolved and removed from metal surfaces.
Choosing a cheap cleaner or using the wrong chemistry type may lead to hidden costs later: poor cleaning results, rework, corrosion, and shorter bath life. A well-formulated product, on the other hand, ensures effective degreasing at lower concentrations and often at lower temperatures — improving both sustainability and performance.
Modern industrial cleaning chemicals and parts cleaners are designed to support energy efficiency. Low-temperature chemistries, for example, can achieve excellent cleaning results while significantly reducing energy consumption and CO₂ emissions.
2. Temperature – finding the sweet spot
Temperature plays a major role in how fast chemical reactions occur and how well soils are removed. However, higher is not always better. While a higher temperature can speed up cleaning, it also increases energy consumption, accelerates chemical breakdown, and can even lead to premature bath ageing.
Optimising temperature means finding the sweet spot — high enough to support cleaning, but low enough to protect the bath and reduce energy costs. With modern low-temperature chemistry, it’s possible to clean effectively at significantly lower temperatures than traditional setups, supporting both process stability and sustainability goals.
3. Time – the efficiency factor
Cleaning time determines how long your parts remain in contact with the cleaning solution. Longer cycles can improve cleaning results, but they also reduce throughput and productivity.
If chemistry and temperature are well-optimised, you can often shorten cleaning cycles without compromising quality. Continuous monitoring and bath maintenance are key to ensuring that shorter cleaning times still deliver consistent, high-quality results.
4. Mechanics – the power of motion
The mechanical aspect refers to how physical force helps remove contaminants — for example through spray nozzles, agitation, brushing, ultrasound, or flowjet systems.
Proper mechanical action improves the efficiency of the entire process. Even the best cleaning chemistry will struggle if the spray pattern is incorrect or the nozzles are clogged. Conversely, optimised mechanical action can reduce the need for higher temperatures or stronger chemicals, creating a more balanced and cost-effective system.
In short, every cleaning process is a balancing act. If you change one parameter, you must compensate with another to maintain cleaning quality. By understanding the relationship between chemistry, temperature, time, and mechanics, you can design a process that delivers cleaner parts, longer bath life, and lower overall costs.
Not sure if your bath chemistry or temperature are balanced?
Why is ‘cheap’ cleaning more expensive?
At first glance, using a low-cost detergent or running your parts washer at higher temperatures might look like an easy way to save time or money. But when you look closer, the picture changes. In industrial cleaning, every shortcut has a price – and it often shows up later as downtime, reduced bath life, or costly rework.
In other words, what looks like a small saving on chemicals can quickly turn into a much bigger loss when you consider the total cost of ownership (TCO). Read more about TCO here.
The hidden costs of ‘cheap’ cleaning
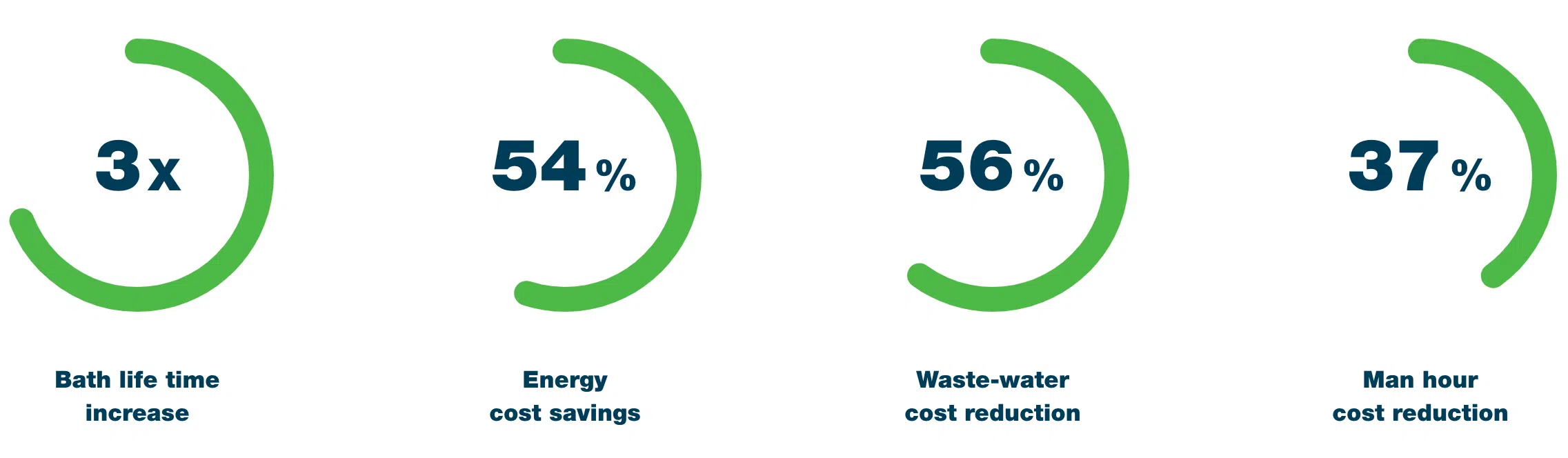
1. Shorter bath lifetime
Low-quality cleaning agents or incorrect dosing often cause the bath to degrade faster. Oils and particles build up more quickly, surfactants are lost, and the chemistry becomes unstable. This leads to more frequent bath changes — meaning more downtime, higher waste disposal costs, and greater environmental impact.
2. Increased energy and water use
Cheaper cleaning products often need higher temperatures or longer wash cycles to perform adequately. That directly increases energy consumption. In some cases, more rinsing is also needed to remove residue, raising water usage and wastewater costs.
3. Equipment wear and corrosion
Unbalanced cleaning processes — especially those with aggressive chemistry or excessive heat — can damage pumps, seals, and other machine components. Over time, that means higher maintenance costs, unplanned downtime, and even premature equipment replacement.
4. Quality issues and rework
If parts are not cleaned properly the first time, the problem quickly spreads down the line. Contaminants that remain on parts can cause coating defects, corrosion, or assembly failures. Each rewash or rejected part adds hidden costs in labour, energy, and lost production time.
5. Environmental and compliance costs
In today’s manufacturing landscape, sustainability is not optional. Excessive energy use, poor wastewater quality, and high chemical consumption all affect your carbon footprint and environmental reporting. Optimising your process isn’t just about cost — it’s about meeting stricter environmental standards and supporting your ESG goals.
The real value of optimisation
The Sinner’s Circle offers a clear roadmap for improvement. Instead of focusing on “cheap” cleaning, focus on smart cleaning — where chemistry, time, temperature, and mechanics are optimised to work together.
By using high-quality, low-temperature cleaning chemistry, you can achieve the same (or better) cleaning results at lower energy use. Balanced parameters extend bath lifetime, reduce waste, and ensure your equipment stays in good condition. Over time, this translates into a lower total cost of ownership and a more reliable, sustainable production process.
When viewed from a TCO perspective, the cheapest solution on paper often turns out to be the most expensive one in practice.
Before cutting costs, make sure you’re not cutting performance.
FAQs Industrial cleaning solutions
What is Sinner’s Circle in cleaning?
The Sinner’s Circle is a model developed by Dr. Herbert Sinner that explains how four parameters — chemistry, temperature, time, and mechanics — work together to determine cleaning performance. In industrial and parts cleaning, balancing these parameters helps you achieve optimal results while reducing energy use, waste, and total cost of ownership.
How does cleaning chemistry impact costs
Cleaning chemistry plays a central role in process efficiency. The right industrial cleaning chemicals or parts cleaning solvent can reduce energy demand, shorten cleaning cycles, and extend bath life. In contrast, low-cost or poorly matched chemistry often leads to instability, higher consumption, and more frequent bath changes — all of which drive up operational costs.
How can I reduce my total cleaning costs?
Focus on optimisation, not reduction. Evaluate all four elements of the Sinner’s Circle together. Lowering temperature or chemical concentration can be effective, but only when balanced with the right time and mechanical action. Modern low-temperature chemistries and continuous bath monitoring help achieve both cost and energy savings.
What role does water quality and bath maintenance play in costs?
Water quality has a major influence on corrosion, foam formation, and overall cleaning stability. Hard water or high chloride levels can reduce bath performance and shorten bath lifetime. Regular bath maintenance — checking pH, conductivity, and surfactant levels — ensures consistent cleaning quality and prevents costly downtime.
Is a stronger chemical always more effective?
Not necessarily. Stronger often means harsher, and that can cause corrosion, surface damage, or safety issues. A balanced, well-formulated product designed for your process will typically deliver better long-term performance and lower overall costs.
Does running at higher temperatures guarantee better cleaning?
No. Higher temperature increases energy consumption and can degrade chemistry faster. The key is to find the optimal temperature that supports cleaning efficiency while protecting your parts, quality, and sustainability goals.
How long should my cleaning cycle be?
There’s no universal answer — it depends on your chemistry, equipment, and contamination type. However, if your bath and mechanical action are optimised, you can often shorten cycles without compromising quality. The goal is consistent, efficient cleaning, not just longer exposure time.
Need an expert eye on your process?
If you suspect your cleaning process could perform better — or cost less — we’re here to help. Our specialists can analyse your setup, review your parameters, and identify optimisation opportunities that extend bath life, improve results, and lower your total cost of ownership.
Read more about related products to this article
Here at DST-CHEMICALS, we continuously work to improve the efficiency and environmental performance of industrial cleaning. Our focus is on developing solutions that reduce energy use, minimise wastewater, and extend bath lifetime — helping customers achieve cleaner parts with fewer resources.